Laboratory Services Nationwide
Established in 2016, London Medical Laboratory is an independent clinical testing specialist and provider of pathology diagnostics services to clients across the healthcare sector.
From our state-of-the-art laboratory near Battersea Park, our experienced team is able to offer tailor made clinical pathology diagnostic services to medical professionals, health care providers and patients across the capital and beyond.
We offer pathology testing solutions across disciplines including clinical biochemistry, immunology, haematology, sexual health screening and molecular biology.
Our facilities and experience ensure we get your clinical test results back to you – fast and hassle free.
We run a laboratory operation and commence processing all samples as soon as they arrive at our laboratory ; thereby ensuring that many results are with you within 4 hours.
London Medical Laboratory is perfectly positioned to help you succeed
- Central London laboratory
- Nationwide coverage
- Comprehensive blood test / health check reports sent to patents next day*
- Patient portal to monitor and track results
1M+ test already
UK's largest phlebotomy network
Led by top medical experts
UKAS Accreditation + CQC Registration
Quality Assurance & Governance
LML delivers a service that is compliant with the requirements for Medical Laboratories set by the United Kingdom Accreditation Service (UKAS: ISO 15189:2012), the Care Quality Commission (CQC), the Health and Safety Executive (HSE) and the Medicines and Healthcare Products Regulatory Agency (MHRA).
Our laboratory is a UKAS accredited medical laboratory (no. 21423) that provides a comprehensive diagnostic service in conjunction with our referral laboratory partners. The laboratory is clinically led and equipped with advanced analytical instruments enabling us to provide fast and accurate results for our customers. All tests offered by LML are enrolled in internationally recognised and accredited quality assurance schemes.
The laboratory is staffed by experience state registered Biomedical Scientists and Medical Laboratory Assistants with specialists in each scientific discipline: haematology, biochemistry, molecular/virology and immunology.
The laboratory continuously adopts emerging technologies to maintain its leadership in clinical diagnostics. Additionally, it regularly contributes to research studies, thereby playing a key role in introducing these innovative products to the market.
Our UKAS accreditation number is 21423 and our electronic Certificate of Accreditation and schedule can be found here.
LML also participates in UK and international assessment schemes such as the National External Quality Assessment Scheme (NEQAS) and the External Quality Assurance Assessment Scheme (EQAAS).
LML is registered with and regulated by the CQC for “Diagnostic and screening procedures” – certificate number: CRT1-8889051055, provider ID: 1-4035892777.
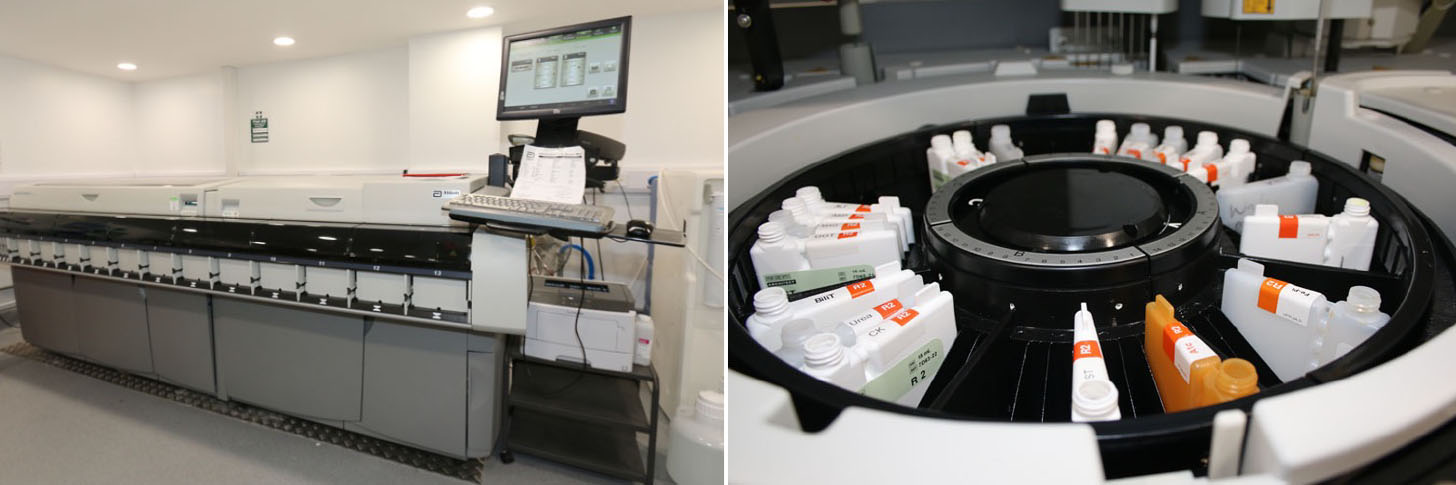
Highly equipped laboratory
Additional Phlebotomy Support & Health Checks
In addition to a complete laboratory service, we offer a phlebotomy service through our extensive network of LML branded partners – many in community pharmacies.
| Services Options | Description |
| In-Store Phlebotomy | Patients can come into one of our stores (LML branches or branded partners) to have their blood taken by one of our qualified phlebotomists. See here (ADD LINK) for locations. |
| At-Home Postal Testing Kits | User-friendly fingerstick blood capillary self-testing kits sent to your patient using a courier or Royal Mail 1st Class Tracked postal service. |
LML also offers a range of health checks. These combine blood tests with clinical measurements and lifestyle questionnaires. Clinicians can choose to administer the Health Check or send patients to one of our locations to have the Health Check administered by a member of the LML team. A report is provided the next day.
Examples of LML home testing kit profiles
- Allergy Complete 295 allergen test
- Cholesterol / Lipid Test
- COVID-19 IgG Antibodies
- Diabetes (HbA1c)
- Erectile Dysfunction Profile
- Female Advanced Sexual Health Screen
- Fertility Hormones Profile
- General Health Profile
- Heart Health Profile
- Iron Status Profile
- Male Advanced Sexual Health Screen
- Male Hormone Profile
- Menopause Hormone Profile
- Pregnancy Test (B-hCG quantitative)
- Progesterone - Day 21 Ovulation
- Prostrate Profile
- Testosterone Check
- Testosterone Plus
- Thyroid Function
- Vitamin B12
- Vitamin D
- Vitamin Profile
LML has its own fulfilment centre allowing us to rapidly pack and distribute kits nationwide. Kits can be tailor-made and branded to suit our clients’ needs. All our kits are IVD registered, CE marked and registered with the MHRA (2020090801184835).
GDPR and Data Security
LML policies cover GDPR, data security and business continuity plans. These policies are available on request. Both of our IT partners (Hicom and Cirdan) are ISO27001 accredited. Under policies defined within our ISO 27001 standards, our partners secure our infrastructure to the highest standards and the IT security landscape is under continual review.
Our environment is hardened to the Centre for Internet Security benchmarks and are subject to regular internal vulnerability tests and external penetration tests.
All environments employ industry leading anti-virus, anti-malware and network protection. Any data entering or leaving the hosting environment is subject to multiple levels of vulnerability detection regardless of the entry/exit point.
We also ensure our hosting and corporate networks are segregated and all perimeters are secured using the latest firewall technology. Access by staff to our networks and servers is controlled through Active Directory and provided on a need-only basis. The hardening policies are enforced using Microsoft Security Compliance Manager.
Our detailed privacy policy covers GDPR and other relevant data and information security processes and policies.
We work with cutting-edge, high-quality products from the following companies





